Medical Device Software Development


Do you struggle to keep up with strict regulatory requirements like HIPAA, HL7, and others and ensure your organization remains compliant?
Our medical device software developers are trained and certified to meet complete regulatory standards. We can create medical software providing automated compliance checks, comprehensive audit trails, and seamless integration with existing systems, ensuring your organization stays ahead of regulatory changes.
Are outdated processes and manual workflows causing inefficiencies, leading to increased workloads for your medical staff and longer wait times for patients?
Our medical software solutions streamline operations by automating routine tasks, improving workflow coordination, and enhancing communication between departments, allowing your staff to focus more on patient care and less on administrative burdens.
If you’re concerned about the challenges of maintaining patient confidentiality and protecting financial information from unauthorized access, data breaches, and cyberattacks, we’re here to help.
Our solutions are designed with robust security measures, such as end-to-end encryption, multi-factor authentication, and strict access controls, to safeguard your patients’ personal and financial information.
Medical Device Software Solutions and Services
Explore the most requested medical device software development modules in Glorium Technologies
Our mobile app solution provides patients and healthcare providers convenient access to medical records, appointment scheduling, data, and telemedicine services. Patients can easily book appointments, receive reminders, and access their health information on the go.
With custom medical device software like our apps module, medical professionals can talk with patients, review medical histories, and offer virtual consultations, enhancing patient engagement and ensuring timely care delivery.

Our advanced analytics module enables healthcare organizations to analyze large volumes of data for actionable insights. It allows organizations to track patient outcomes, identify trends, and make informed decisions based on comprehensive data analysis.
This module also offers predictive analytics to forecast patient needs and resource utilization, helping healthcare providers optimize operations and improve patient care.
Our cloud platform provides secure, scalable storage and management of medical data. Users can easily access patient records, collaborate with other healthcare providers, and ensure data is backed up and recoverable.
The platform supports integration with various healthcare systems, facilitating seamless data exchange and interoperability while adhering to strict security and compliance standards.
Our IoT-based medical device tracking solution allows real-time monitoring and management of medical equipment. Track device location, usage, and maintenance schedules to ensure optimal performance and availability.
This solution also provides alerts for device malfunctions or required maintenance, reducing downtime and improving the efficiency of medical operations.

Our real-time health monitoring modules offer continuous monitoring of patients’ vital signs, such as heart rate, blood pressure, and oxygen levels. Receive immediate alerts for any critical changes, enabling prompt medical intervention.
These modules also provide patients with personalized health insights and trends, fostering proactive management of their health conditions and enhancing overall care quality.
What Makes Us Pros in Mental Health App Development?
Leading Software Examples Benefiting from Integration with Medical Devices
Medical device software development changes and improves existing software and applications, improving your workflow and overall patient experience.
By integrating with medical devices, these apps can provide real-time vital sign monitoring, allowing healthcare providers to make informed decisions during virtual visits and improving diagnostic accuracy and patient outcomes.
Integrating EHRs with medical devices allows automatic data entry from diagnostic tools and monitoring equipment, ensuring up-to-date and accurate patient information.
These apps can provide reminders, monitor adherence, and track health indicators that may influence medication efficacy when integrated with medical devices.
Must-Have Features for Medical Device Software
We include essential features during our development process to ensure your medical device software is user-friendly, effective, and compliant.
Our medical device software solutions are designed to comply with all relevant industry standards and regulations, including HIPAA, HL7, ISO 13485, and FDA guidelines. By adhering to these strict requirements, we ensure that our software meets the highest safety and quality standards.
Additionally, our custom medical device software solutions include comprehensive audit trails that log all actions and changes within the system. These detailed logs support regulatory audits and approvals, providing transparency and accountability and helping healthcare companies effortlessly maintain compliance.
We prioritize the security and privacy of sensitive medical data with end-to-end encryption, protecting information during transmission and storage. This robust security measure ensures that data remains confidential and secure from unauthorized access.
Furthermore, our medical device software includes multi-factor authentication and strict access controls to enhance security. By requiring multiple verification forms and limiting access to authorized personnel only, we protect against potential breaches and unauthorized data access.
Our software supports standards-based integration, enabling seamless connectivity with other healthcare systems and devices using protocols. This ensures smooth data exchange and operational continuity across different platforms.
We also provide robust APIs and Software Development Kits (SDKs) for easy integration with other software and hardware. This flexibility allows healthcare companies to efficiently enhance their existing systems and incorporate new technologies.
Our software features an intuitive design that makes it easy for both medical professionals and patients to navigate. By focusing on user experience (UX) and user interface (UI), we ensure that users can quickly and easily access the necessary information.
In addition, our solutions are optimized for various devices, including desktops, tablets, and smartphones. This responsive design allows users to access the software from any device, improving accessibility and convenience.
Our medical device solutions enable real-time data access, allowing healthcare providers to instantly monitor and analyze patient data. This capability ensures that critical health information is always up-to-date and available when needed.
Additionally, we provide instant alerts and notifications for critical health changes or medical device malfunctions. These timely alerts enable prompt medical intervention, improving patient outcomes and safety.
We incorporate advanced analytics tools into our software, allowing healthcare firms to analyze large volumes of data for actionable insights. These tools help identify trends, predict patient needs, and support informed decision-making.
Our solutions also offer customizable reports, enabling users to generate and tailor reports based on specific needs. This feature helps healthcare professionals track performance, monitor outcomes, and improve overall efficiency.
Our software supports remote monitoring, enabling healthcare providers to monitor patients’ health from a distance. This feature is handy for managing chronic conditions and ensuring continuous care.
Additionally, our telemedicine integration allows for video consultations and virtual care, making healthcare more accessible. This integration enhances patient engagement and ensures timely medical attention, regardless of location.
We offer workflow features that streamline routine tasks and enhance operational efficiency. By automating processes, healthcare providers can focus more on patient care and less on administrative tasks.
Our software also ensures seamless data transfer between the system and Electronic Health Records (EHR). This integration eliminates data entry redundancies, reduces errors, and improves overall workflow efficiency.
Our software is designed with a modular architecture, making it easily scalable and customizable to meet the evolving needs of healthcare organizations. This flexibility allows for the addition of new features and capabilities as needed.
Additionally, we provide cloud-based solutions that enhance scalability and remote access. This cloud support ensures that healthcare professionals can access the software and data anywhere, improving efficiency and collaboration.
We conduct comprehensive testing, including unit, integration, and user acceptance testing (UAT), to ensure the reliability and performance of our software. This rigorous testing process helps identify and resolve issues before deployment.
Moreover, we implement thorough validation processes to confirm that our software meets all functional and regulatory requirements. This ensures our solutions are safe, effective, and compliant with industry standards.
We provide extensive user support and training to help healthcare professionals maximize the potential of our software. Our support includes comprehensive user manuals and online help resources that offer detailed guidance and troubleshooting.
In addition, we offer training programs that cover all aspects of the software, ensuring that users are well-equipped to utilize its features effectively. This training helps healthcare companies achieve smooth implementation and operation.
Our software includes robust data backup features to ensure regular and secure backups of all critical information. This backup capability protects against data loss and ensures that patient records are permanently retrievable.
We also develop and implement comprehensive disaster recovery plans to maintain data integrity and availability in emergencies. This ensures that healthcare professionals can quickly restore operations and continue providing care without significant disruptions.
Our Certifications
As a leading medical device software development service provider with over a decade of industry experience, we ensure that the custom solutions we deliver to clients worldwide are secure and compliant with all industry regulations. Our expertise guarantees that your medical software meets the highest safety, reliability, and performance standards.
- Industry-wide standards including HIPAA, HL7&FHIR, and GDPR.
- ISO 9001 certification for Quality Management.
- ISO 13485 certification for Quality Management of Medical Devices.
- ISO 27001 certification for Information Security Management System.
Consultation and Analysis
We start developing medical device software with a consultation to understand your needs and goals. During this stage, our team gathers detailed requirements and analyzes functional, technical, and regulatory aspects of your medical device project.
Design and Prototyping
After the analysis we develop prototypes to visualize the medical device software and gather feedback from stakeholders. This iterative design process is essential to ensure that the medical software aligns with your vision and meets all functional and user requirements.
Development Process
Our team of experts then proceeds with medical software development, building robust and scalable solutions. We integrate necessary components, including medical devices, to ensure seamless functionality and interoperability. This step involves coding, unit testing, and integrating various medical functions to create a cohesive and reliable system.
Testing and Validation
After your medical device software is developed, we start testing to ensure it meets all regulatory standards and performs flawlessly. Our validation process includes functional, performance, and user acceptance testing to identify and resolve any issues.
Regulatory Compliance
We ensure your medical device development project complies with industry regulations and standards. We provide detailed documentation and support for regulatory submissions, ensuring that your medical software is fully compliant and ready for market approval. This step is crucial for maintaining the safety and efficacy of the medical devices.
Deployment and Support
Finally, we deploy the medical device app development solution and offer ongoing support to ensure smooth operation. Our post-launch services include maintenance, updates, and user training to help you maximize the benefits of your medical software.
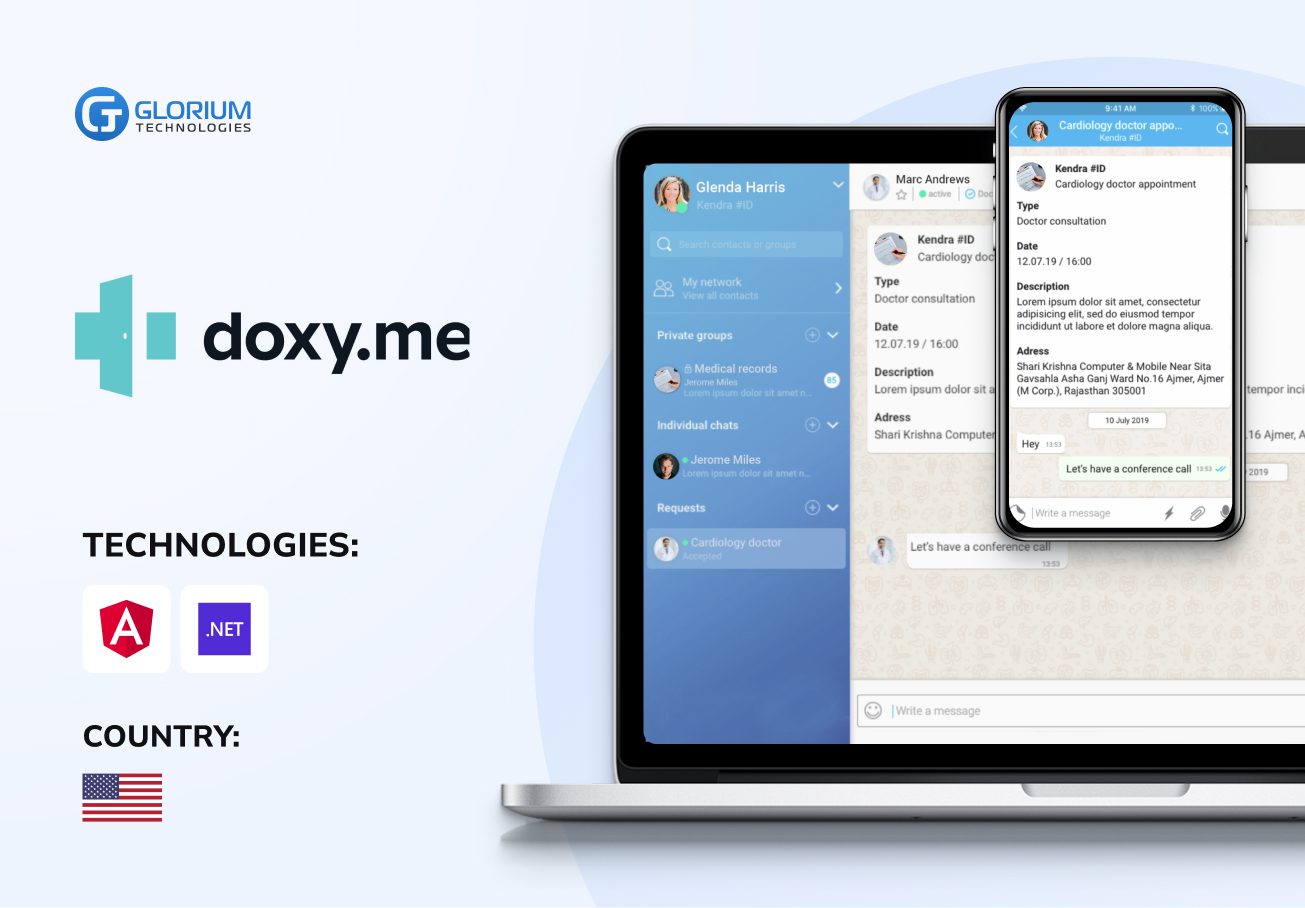
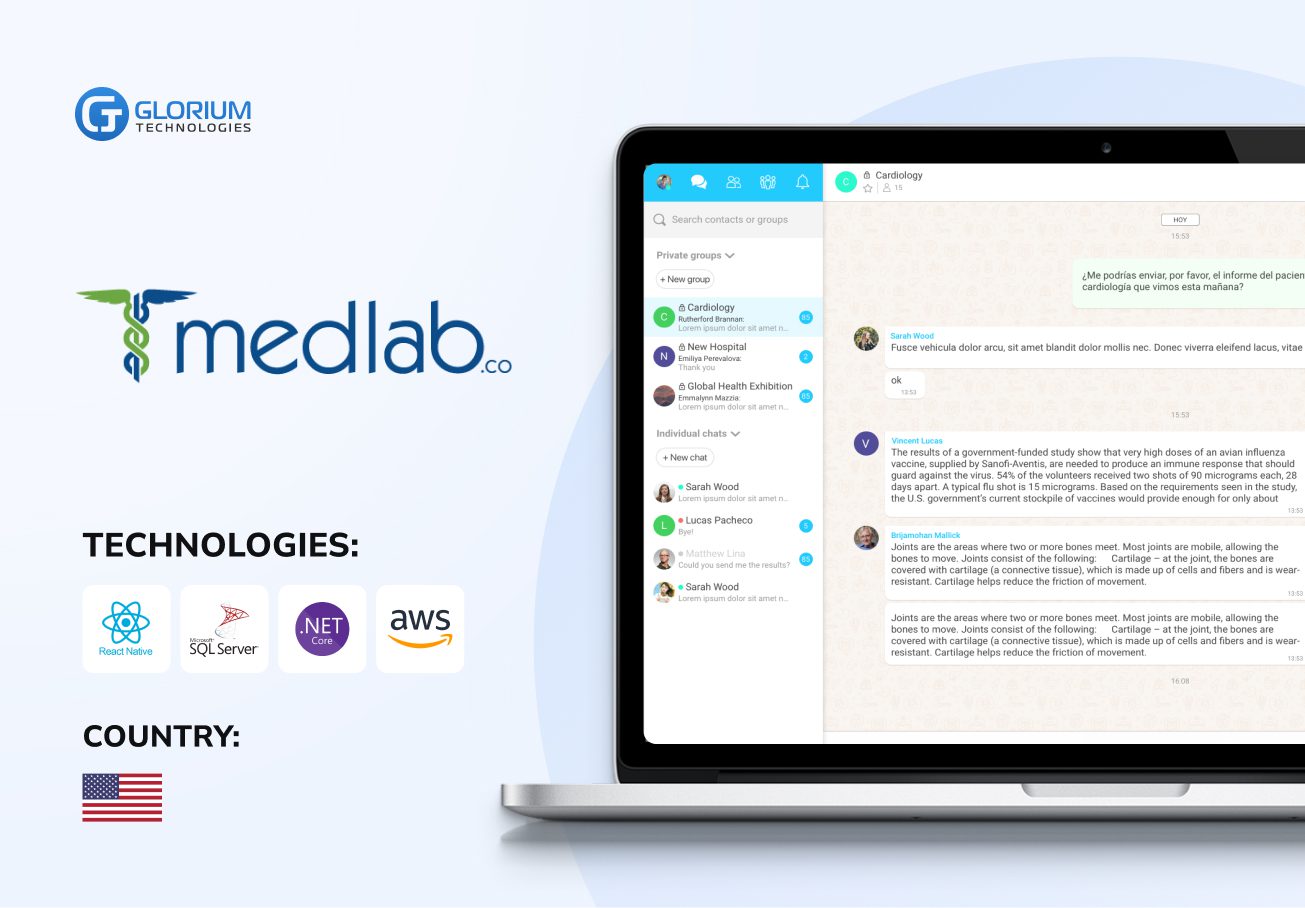
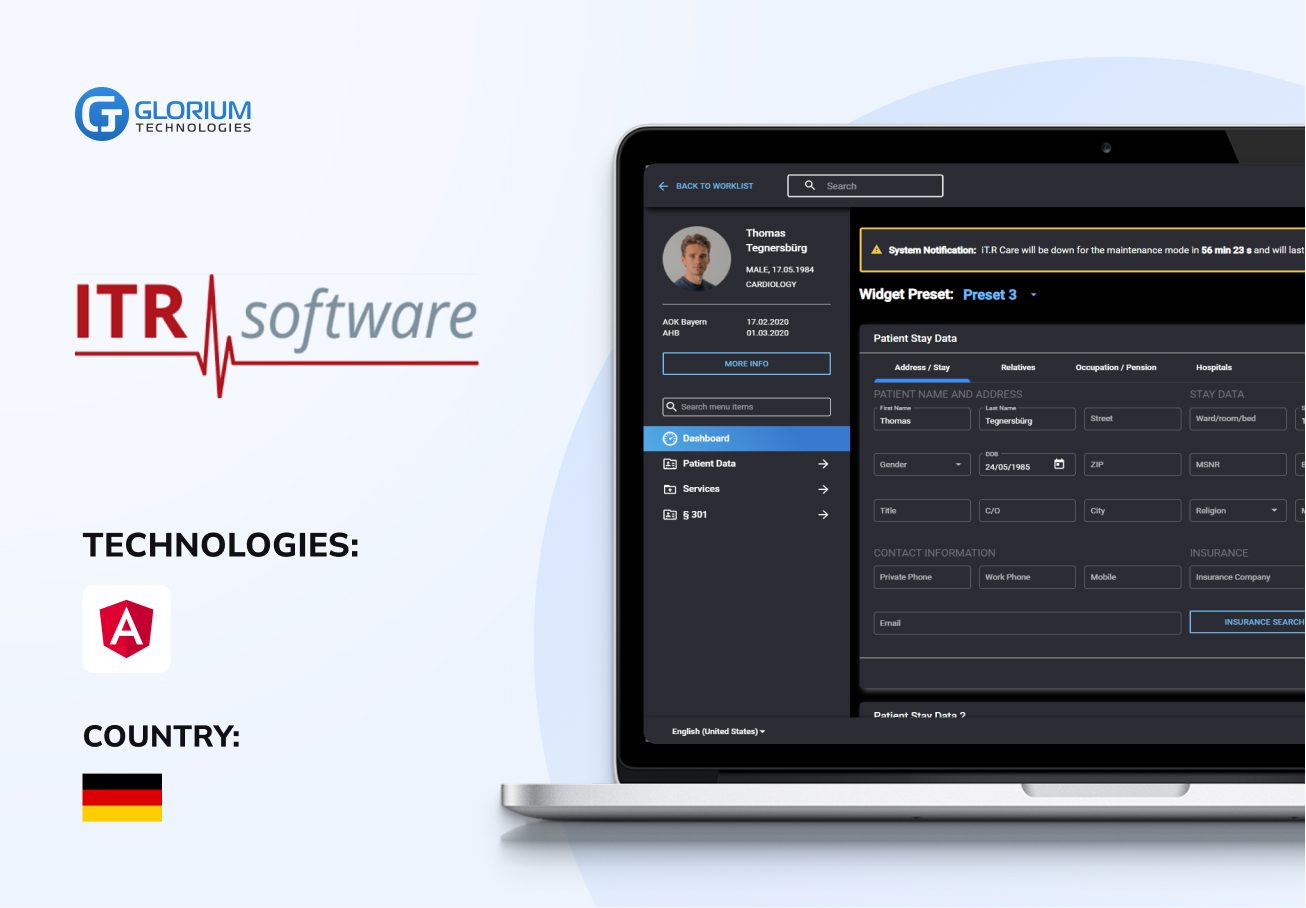
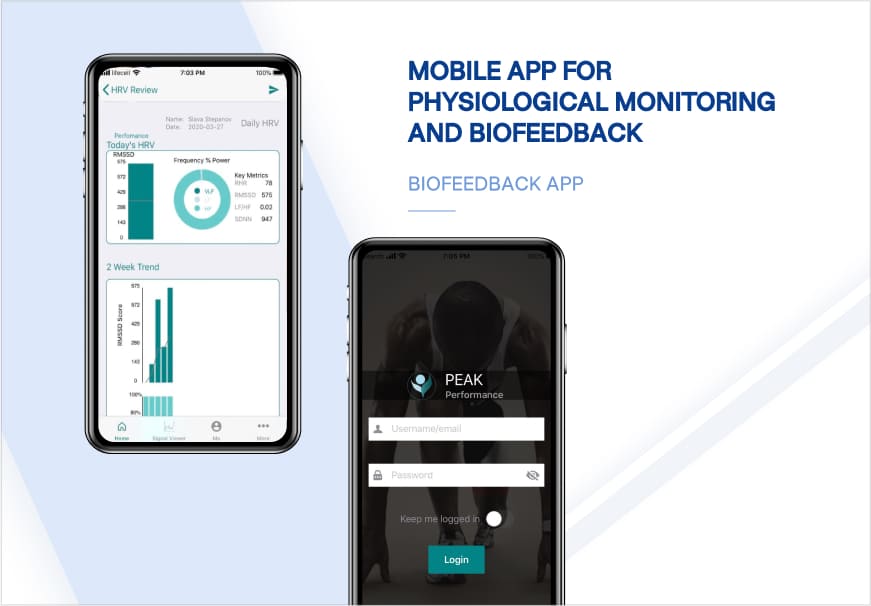
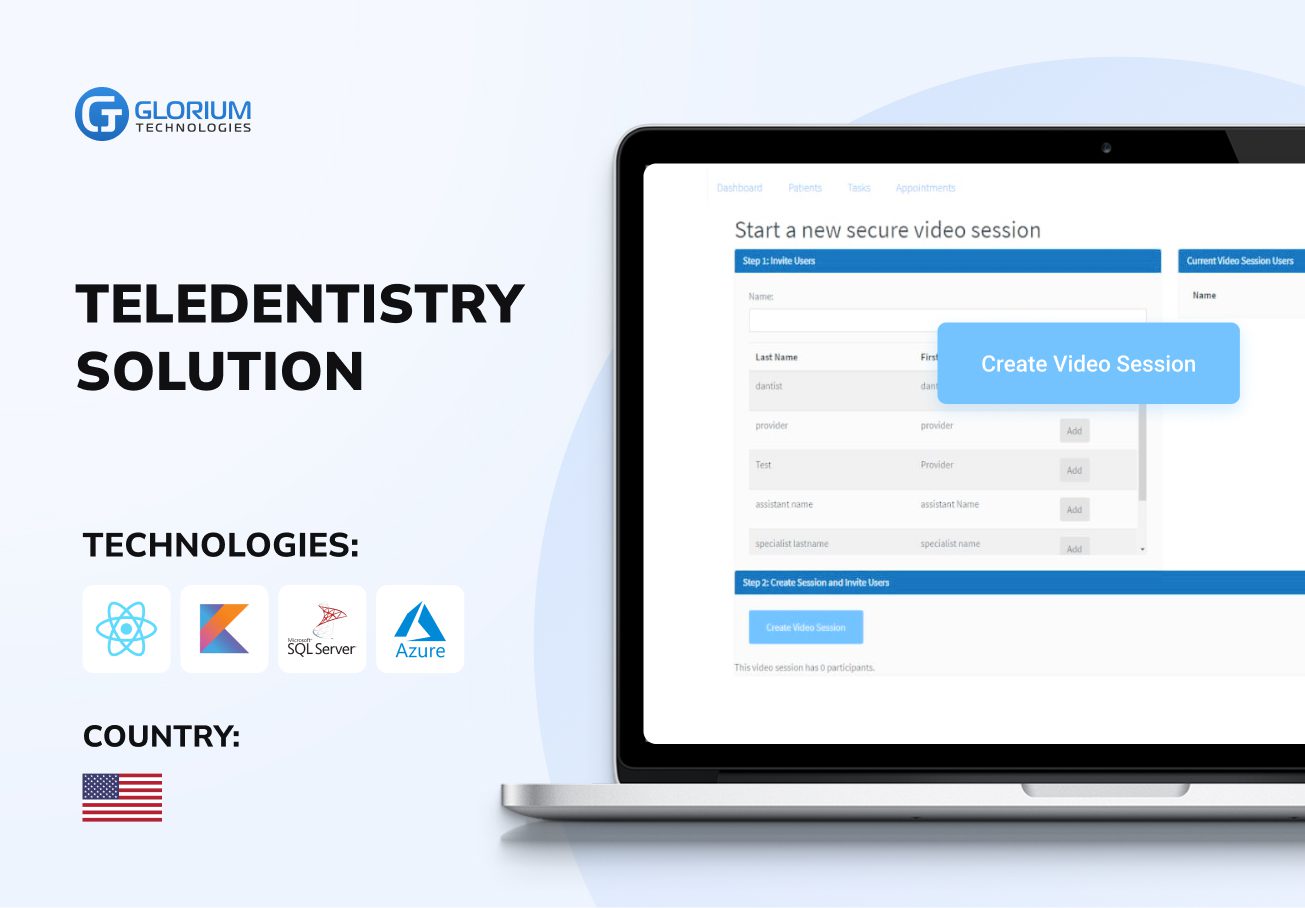
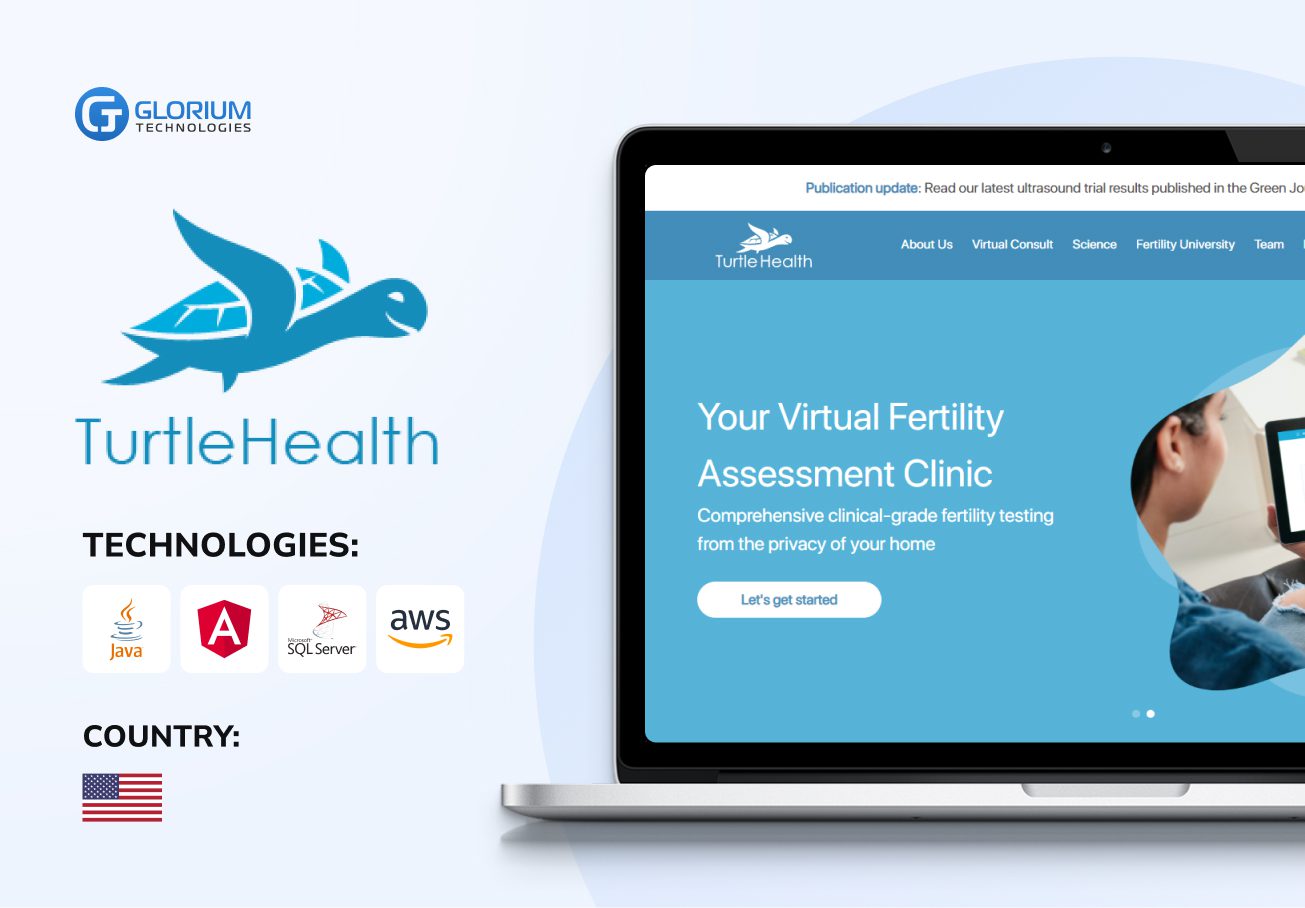
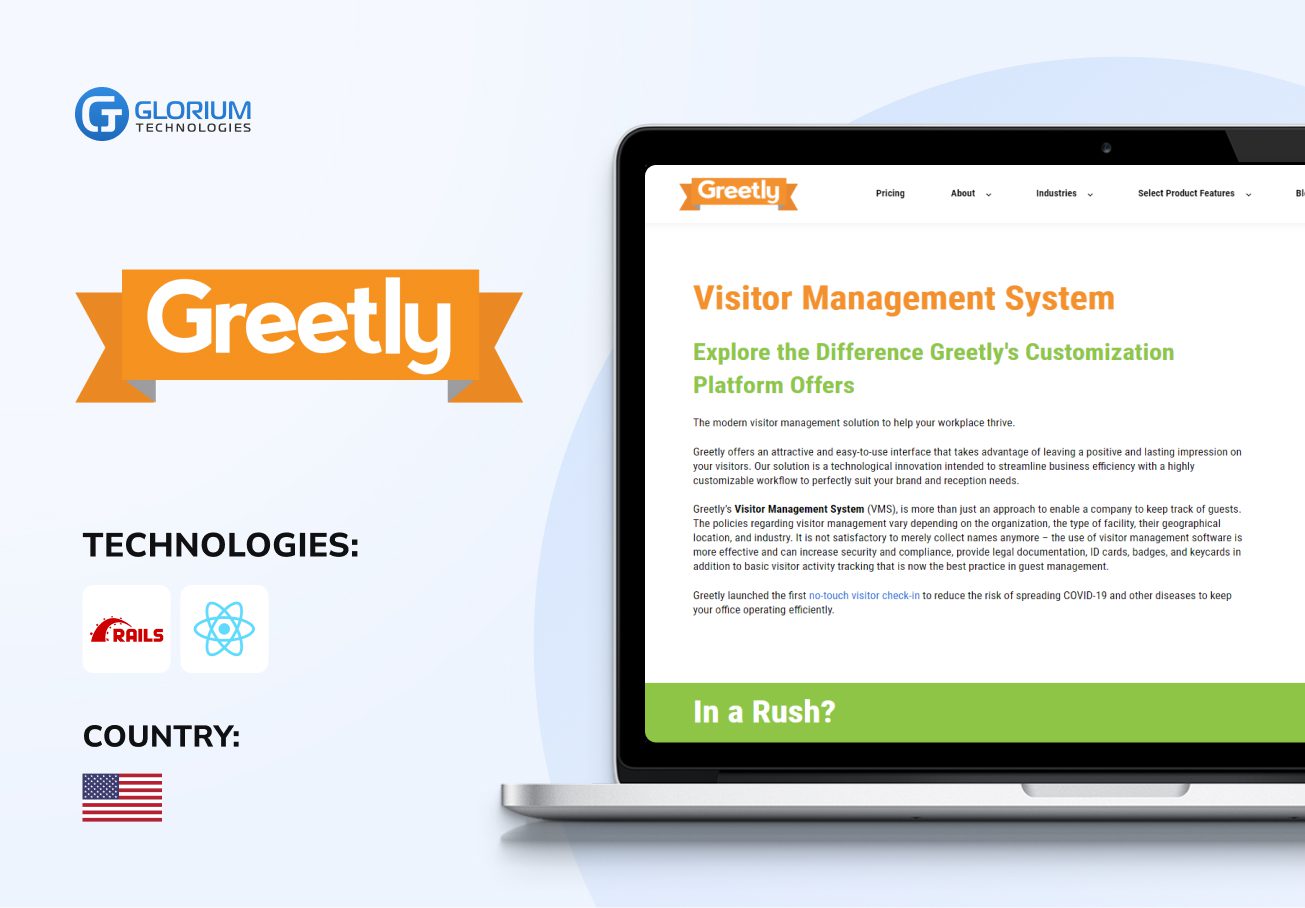
Recent healthcare clients






Clients say about us
Some Clients' Journeys
Who we are
Read moreGlorium Technologies is a full-cycle app & software development company which covers specific client business needs and manage them with the help of the best possible technology solutions.
Since 2010, we have been inventing digital breakthroughs, helping startups and businesses come out on top in their markets.
Why choose us
Building Custom Medical Device Software: Best Practices and Tips
From herbal remedies to rudimentary surgeries and age-old healing practices, the healthcare industry has evolved remarkably. It’s a development process that goes on infinitely, offering us new solutions and practices and seamlessly blending with the development of the world and society.
Today, the integration of digital technologies, such as electronic health records, telemedicine, and AI-powered diagnostics, has revolutionized patient care, making it more efficient, personalized, and accessible. This journey from primitive practices to cutting-edge innovations reflects the relentless pursuit of improving health outcomes and the quality of life for people worldwide.
Healthcare firms and providers opt for smaller, portable, accessible medical devices. These devices can even reach patients’ homes and streamline their healthcare experience. Of course, we’re talking about medical device software development.
Medical devices improve patient care and streamline workflow, making it easier for organizations to operate and care for their patients.
In this comprehensive guide, we’ll explore medical device software engineering, its benefits, risk management, and best practices.
What is Medical Device Software?
Medical device software is designed for medical purposes, such as diagnosing, preventing, monitoring, or treating diseases. It can function independently or in combination with other medical devices, and it must comply with strict regulatory standards to ensure safety and efficacy.
Think of medical device software as the brain of modern medical devices. Just like how your brain processes information and helps you make decisions, this software analyzes data from medical devices and provides crucial insights to healthcare professionals.
For instance, it can help a doctor make an accurate diagnosis by interpreting the results from an MRI scan.
Medical device software is becoming increasingly popular and essential in modern healthcare due to its ability to enhance diagnostic accuracy, improve patient monitoring, and streamline treatment processes.
Healthcare firms slowly but surely adopt this advancement to provide personalized care and:
- Enhance patient care and safety through accurate diagnostics and monitoring
- Ensure compliance with regulatory standards, reducing legal and financial risks
- Improve operational efficiency by automating and streamlining clinical workflows
- Facilitate the integration of advanced technologies like AI and IoT into healthcare practices
- Provide scalable solutions that can evolve with technological advancements and healthcare needs
Two Types of Software for Medical Devices
As the healthcare industry develops and medical devices get smarter and more intelligent, we underline the two main types of device software – embedded medical systems and software as a medical device (SaMD).
Embedded Medical Systems
Embedded medical systems are integrated within medical devices to perform dedicated functions, often critical to the operation of the device. These systems consist of hardware and software components designed to work seamlessly together to monitor, diagnose, and treat medical conditions.
Some examples of embedded systems in medical devices you’ll recognize include MRI and CT scanners. They use embedded device software to process and display detailed images of the body’s internal structures.
Other interesting examples include:
- Defibrillators: They monitor heart rhythms and deliver shocks if an abnormal heartbeat is detected, and blood glucose monitors provide real-time blood sugar levels for diabetes management
- Insulin pumps: These pumps continuously monitor blood glucose levels and adjust insulin delivery accordingly, helping patients manage their diabetes more effectively and with greater precision
- Fetal heart monitors: These monitors track a baby’s heart rate during pregnancy and labor, providing critical data to ensure the health and safety of both the mother and baby
Software as a Medical Device (SaMD)
Software as a Medical Device (SaMD) performs medical functions without being part of a hardware medical device. SaMD can run on general-purpose platforms like smartphones, tablets, or cloud servers and is used for various purposes, such as diagnosis, treatment recommendations, or patient monitoring.
This device software can analyze medical data, support clinical decisions, and provide treatment recommendations based on real-time patient information.
Some notable SaMD examples are:
- Mobile apps that analyze MRI images: They detect abnormalities or software that uses artificial intelligence to predict patient outcomes based on historical data
- AI-driven diagnostic apps: These apps analyze medical images like X-rays and MRIs to detect diseases such as cancer at an early stage, thereby improving diagnostic accuracy and patient outcomes
Benefits of Medical Device Software Development
Medical device development is costly and time-consuming, so why do healthcare companies plan to develop custom medical device software? The myriad benefits of robust and reliable software for these critical devices make it worthwhile.
Enhanced Patient Care
Medical device software development directly improves patient care by providing precise, real-time data that aids diagnosis and treatment. For example, software in wearable devices can continuously monitor vital signs like heart rate and blood glucose levels, sending alerts to healthcare providers if something goes awry.
This kind of continuous monitoring can catch potential health issues early, allowing for quicker and more effective interventions.
Moreover, medical device software often includes patient management tools that help doctors and nurses keep track of patient histories, medication schedules, and treatment plans.
These features streamline communication and ensure that everyone involved in a patient’s care is on the same page, reducing the chances of errors and improving overall patient outcomes.
Improved Operational Efficiency
One of the most significant benefits of a medical device software project is its operational efficiency boost. By automating routine tasks such as data entry and patient monitoring, medical device manufacturers can significantly reduce the workload on healthcare professionals. This allows them to focus more on patient care and less on administrative duties.
Additionally, integrated health tech solutions can streamline workflows in hospitals and clinics. For instance, software that links different departments—such as radiology, lab services, and patient records—ensures that all necessary information is available instantly, eliminating delays and improving the speed and accuracy of medical services.
Regulatory Compliance
Medical device software development also helps companies stay compliant with regulatory standards. Regulatory bodies like the FDA and the European Medicines Agency have stringent requirements for medical devices, including software components.
A well-designed software solution ensures that all aspects of the device meet these regulations, from data security to patient safety.
Compliance with these standards not only avoids legal troubles but also builds trust with users and healthcare providers. It assures them that the medical device has undergone rigorous testing and meets the highest safety standards, which is crucial for both market acceptance and patient safety.
Customization and Scalability
Another significant advantage is the ability to customize and scale solutions to meet specific needs. Medical device software can be customized to fit the unique requirements of different healthcare settings, whether it’s a small clinic or an extensive hospital network.
This flexibility ensures the software can adapt to various workflows and patient management systems. Scalability is equally important, especially as medical technology evolves. As new features or updates are needed, scalable software can accommodate these changes without requiring a complete overhaul.
This future-proofs the investment and ensures that the medical devices remain up-to-date with the latest technological advancements.
Enhanced Data Security
Data security is especially essential in digital healthcare. Medical device software projects prioritize protecting sensitive patient data from breaches and unauthorized access. Advanced encryption methods, secure access controls, and regular security updates are integral to these software.
By ensuring robust data security, medical device manufacturers protect patients’ personal information and build trust with healthcare providers. Secure software also helps in compliance with data protection regulations like HIPAA in the U.S. and GDPR in Europe, further safeguarding against potential legal issues.
Innovation and Competitive Edge
Investing in medical device software development fosters innovation, giving companies a competitive edge. Advanced solutions can integrate AI and machine learning to enhance diagnostic accuracy and treatment personalization.
For example, AI-powered software can analyze complex medical images faster and more accurately than human clinicians, leading to quicker and more accurate diagnoses.
Staying at the forefront of technology not only attracts more clients but also positions the company as a leader in the medical device industry. This reputation can lead to increased market share and opportunities for growth, making it a worthwhile investment for medical device manufacturers.
How to Develop a Medical Device Software: A Step-by-Step Guide
Developing medical device software is a complex process that requires strict regulatory guidelines and rigorous testing procedures to ensure patient safety and product quality.
Here’s a step-by-step guide to help medical device manufacturers navigate this process:
Define Requirements and Objectives
First, you need to identify user needs and understand what they’re looking for in your software. Conduct surveys, interviews, and focus groups to gather detailed requirements.
This essential step helps set clear objectives, including specific functions, performance criteria, and compliance with regulatory standards.
Plan and Design
In the second stage, you need to develop a project plan and outline the timeline and milestones for the medical device software project. Include risk management plans and contingency measures.
Create a detailed software architecture that outlines how different components of the software will interact. This includes database design, user interfaces, and integration points with other systems.
Development
After you build a prototype to visualize the design and gather feedback from stakeholders, it’s time to choose the right tools and tech stack. Select appropriate programming languages, frameworks, and development tools that align with your project requirements and regulatory standards.
Implement coding standards, version control, and continuous integration practices to ensure code quality and maintainability.
Testing and Validation
Once your development team has the software ready, it’s time to test and validate it. Test individual components of the software to ensure they work as expected. This includes both functional and non-functional testing.
Verify that the software’s different modules and components work together seamlessly. This is crucial for identifying issues when integrating various system parts.
Don’t forget about the compliance testing. Ensure the software complies with all relevant regulatory standards and guidelines, such as ISO 13485, FDA, and EU MDR.
Regulatory Approval
Create detailed documentation covering all aspects of the software development process, including design, development, testing, and validation. This documentation is critical for regulatory submissions.
Submit the required documentation to relevant regulatory bodies for approval. This may include pre-market submissions, risk assessments, and clinical evaluations.
Deployment and Maintenance
Finally, approved software will need deployment and continuous maintenance. Ongoing support is necessary to address post-deployment issues, fix bugs, and ensure compliance with regulatory standards.
Continuous maintenance is one reason many healthcare companies hire professional teams to develop custom medical device software. Thanks to their experience, they help maintain the software’s effectiveness and reliability.
If you’re looking for a professional team for your medical device software, contact us today for a project scope and a free consultation.
How Can Glorium Technologies Help You Develop Medical Device Software
Choosing the right medical device software company is crucial for the success of your project. Glorium Technologies is a full-cycle app and software development company with 200+ skilled professionals, 12+ years on the market, and a 99% client satisfaction rate.
Contact us today and book a free consultation with our representative. Leverage our in-depth industry experience, fully customized solutions, and a user-centric approach.
What programming languages are used in medical devices?
Several programming languages are commonly used in the development of medical devices, each chosen for its specific strengths:
- C and C++: Ideal for embedded systems due to their performance and hardware control.
- Python: Used for data analysis, machine learning, and interfaces.
- Java: Suitable for cross-platform applications.
- MATLAB: Used for algorithm development and signal processing.
- Assembly Language: Employed for low-level programming in devices like pacemakers.
- C#: Common for developing applications on Windows platforms.
What are the types of medical device software?
Medical device software falls into several categories. Embedded medical software, like MRI machines and insulin pumps, is built directly into medical devices to control their functions. Another type, Software as a Medical Device (SaMD), operates independently to perform medical tasks such as diagnostics or health monitoring. Additionally, software in medical devices (SiMD) works in conjunction with hardware to enhance the functionality of medical equipment.
How long does it take to develop a medical device?
Developing a medical device typically takes between 3 to 7 years. This timeframe includes research, design, testing, regulatory approval, and manufacturing. The exact duration can vary based on the complexity of the device and regulatory requirements.
What are some examples of software used in medical devices?
Examples of software used in medical devices include the control systems in insulin pumps, firmware in pacemakers that manage heart rhythms, and the software in MRI machines that process and display images.